On this website, Dr Pascal Vrticka would like to provide an introduction into the most important issues researchers are facing when planning and carrying out fNIRS hyperscanning studies.
Please feel free to get in touch with Dr Vrticka if you have any questions, remarks and/or ideas on how to extend this content as well as how to ensure its wider dissemination as part of workshops, webinars etc. – see here for Dr Vrticka’s contact information.
Another great fNIRS Resource Manual including training videos for NIRx NIRSport2 system users has furthermore been made available by Dr. Ece Demir-Lira’s Development, Experience and Neurocognition (DEN) Lab (University of Iowa).
The below content was / will be presented in detail at the following events as part of interactive workshops, webinars etc.:
May 2023
Functional near-infrared spectroscopy (fNIRS) hyperscanning workshop held at the Department of Psychology, University of Essex (Colchester, United Kingdom). The workshop was supported by Trinh Nguyen (Italian Institute of Technology, Rome), the SoNeAt Lab & Artinis Medical Systems, and hosted by SIRG SoNeAt as part of SEAS.
August 2023
10th International Summer School in Biomedical Engineering held at the Max Planck Institute for Human Cognitive and Brain Sciences (Leipzig, Germany).
The following freely accessible recordings of Dr Vrticka’s introduction to fNIRS hyperscanning presented at the above events are available:
NIRx Webinar Part I
NIRx webinar series on fNIRS Hyperscanning
fNIRS Hyperscanning: Underlying Theory and Experimental Design
fNIRS Hyperscanning – An Introduction
Outline:
1. What is fNIRS Hyperscanning?
2. What is the main measure derived from fNIRS hyperscanning?
3. How do I choose the best optode template?
4. How do I ensure that I obtain high quality fNIRS hyperscanning data?
5. Which data analysis steps does fNIRS hyperscanning involve?
6. How is interpersonal neural synchrony derived from fNIRS hyperscanning data?
7. What about control analyses?
8. What else do I need to keep in mind?

1. What is fNIRS hyperscanning?
Broadly speaking, hyperscanning refers to the simultaneous measurement of physiological signals from at least two participants who are engaging with the same task or stimulus. The term “hyperscan” was coined by Montague et al. (2002) and specifically refers to measuring the neural substrates of human social interaction by means of functional magnetic resonance imaging (fMRI) hyperscanning. Since then, the concept of hyperscanning has been extended to also include other neuroimaging methods, including electroencephalography (EEG; e.g., see Dumas et al., 2010), functional near-infrared spectroscopy (fNIRS; e.g., see Cui et al., 2012) and magnetecephalography (MEG; e.g., see Baess et al., 2012). Moreover, hyperscanning also considers behavioural data (e.g., touch, eye gaze, vocalisations, etc.), peripheral physiology data (e.g., heart rate, etc.) and endocrinology data (e.g., secretion of hormones such as cortisol, oxytocin, etc.) within the greater realm of bio-behavioural synchrony (BBS; e.g., see Feldman, 2017).
fNIRS hyperscanning more specifically uses infrared light absorption to indirectly measure brain activation based on changes in both oxy- and deoxyhemoglobin (HbO and HbR, respectively) – see Figure “fNIRS Hyperscanning Setup” below. fNIRS is thus most closely related to fMRI. However, fNIRS has several advantages over fMRI (and other neuroimaging methods), including its relatively low cost, strongly reduced susceptibility to movement artefacts and greatly enhanced ecological validity. fNIRS therefore is particularly well suited for studies involving infants and children and allows for the assessment of social interactions in much more naturalistic settings – especially when employing the the newest portable / wireless fNIRS devices.


2. What is the main measure derived from fNIRS hyperscanning?
The main measure derived from fNIRS hyperscanning is synchrony. Synchrony is defined by Oxford Languages as “simultaneous action, development, or occurrence“. When employing fNIRS, synchrony is observed at the neural level and thus as the correlation of brain activity across two or more individuals over time during and shortly after social interaction, also known as interpersonal neural synchrony (INS) or interbrain synchrony (IBS).
Importantly, the concept of INS differs somewhat from the general definition of synchrony provided above – as INS does not require the individual brain activation signals to be occurring simultaneously or to be exactly the same. In other words, there can be high INS not only if both individual singals are “in sync” (i.e., in phase), but also if there is a consistent phase shift (i.e., one signal always precedes or follows the other), or even if the two signals are in opposite or anti-phase. The determining variable underlying INS therefore is temporal coherence or contingency (for more details, see also “6. How is interpersonal neural synchrony derived from fNIRS hyperscanning data?” below).
3. How do I choose the best optode template?
Before any fNIRS hyperscanning data can be acquired, the most suitable optode template needs to be chosen. The optode template is the arrangement of emitters and receivers that are going to be placed on participants’ heads. All fNIRS data acquisition software packages have pre-installed optode templates amongst which a suitable one can be picked. Some software packages even have optode template builders that can be used to create custom arrangements. As a rule of thumb, as long as the inter-optode distances are kept at 30 mm, and associations between emitters and receivers remain consistent with the template, templates can be freely rotated to cover the desired brain area(s).
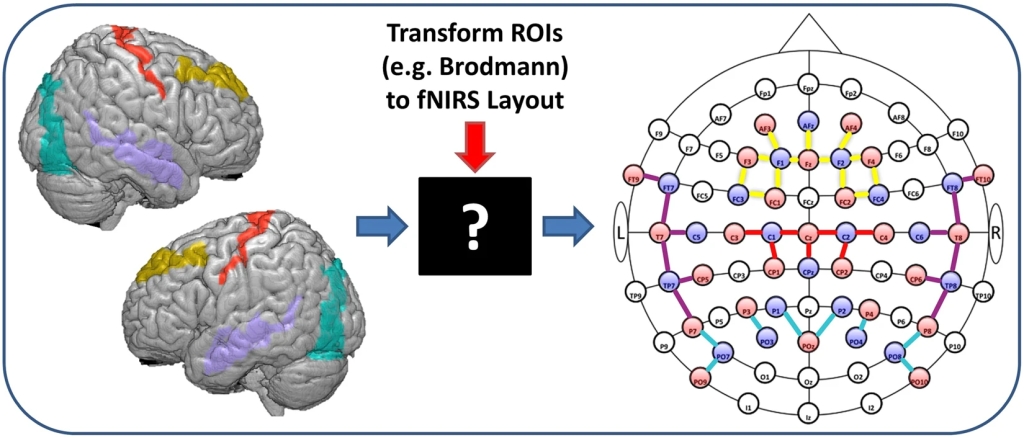
A common challenge that fNIRS (hyperscanning) researchers are facing is the translation of study hypotheses to an fNIRS optode layout by choosing appropriate sources and detectors positions to maximize anatomical specificity to regions of interest. One approach commonly used to tackle this challenge is to refer to the EEG 10/20 system and place optodes relative to EEG electrode sites (which often are printed on the fNIRS caps). Another approach is to use the fNIRS Optodes’ Location Decider (fOLD) toolbox – see Figure “fOLD Toolbox” below (taken from here). The fOLD toolbox automatically decides the location of fNIRS optodes from a set of predefined positions with the aim of maximizing the anatomical specificity to brain regions of interest. Please see here for a webinar by NIRx Medical Technologies about “devfOLD: A Toolbox for Designing Age-Specific fNIRS Channel Placement“.
According to the selected optode template, researchers then need to decide whether they can fit it to pre-punched caps provided from the manufacturer, or whether they need customised caps with holes punched at specific locations. Customisation can either be requested from the manufacturer or caps without holes can be ordered and the punching can be carried out at the researchers’ lab.

4. How do I ensure that I obtain high quality fNIRS hyperscanning data?
Good individual signal quality is key for fNIRS hyperscanning. If data from one channel in one participant is bad, data from this channel also needs to be discarded for the other participant within the same dyad. It therefore is vital to perform a proper signal calibration before starting the fNIRS data acquisition.
Most current fNIRS data acquisition software packages include a simple visual display of signal quality for each measurement channel (e.g., green = good, orange = medium, red = bad). Depending on the manufacturer, different steps can be taken, should signal quality be poor. In some cases, signal gain can either be de- or increased and optodes can be placed deeper or higher in the optode holders, and thus closer to or further away from the head. Often, bad signal is due to the infrared light transmission being obstructed by hair and thus any hair should be carefully moved to the side at each affected optode location.
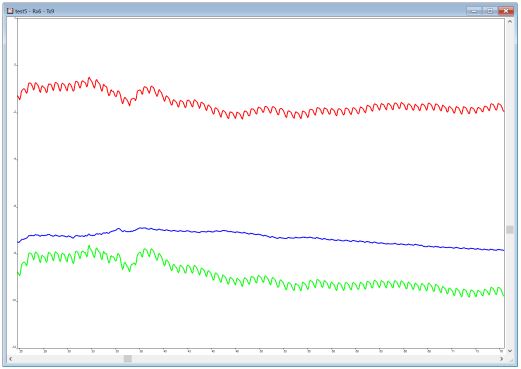
Checking signal quality during calibration can also be done by directly relying on the HbO and HbR signal time courses displayed by the acquisition software. When doing so, a clear heartbeat should be visible in the HbO but only minimal/no heartbeat in the HbR signal (see Figure “Optimal Signal” below). Large peaks / signal deflections in both the HbO and HbR signals are indicative of optode movement relative to the head, which can be improved by tightening the cap. A lot of noise in both signals usually points to little / no light being detected by the receiver, which may be due to hair obstructing infrared light transmission. Conversely, no detectable change in both signals usually means that maximum light intensity is detected by / reaching the receiver and thus that the emitter and / or receiver are not well placed within caps relative to the head surface. It could also be that there is too much ambient light being captured by the receiver. Adjusting optodes and / or using an overcap may be advisable.
Please also see here for a webinar by NIRx Medical Technologies on “Optimizing fNIRS Signal Quality”.

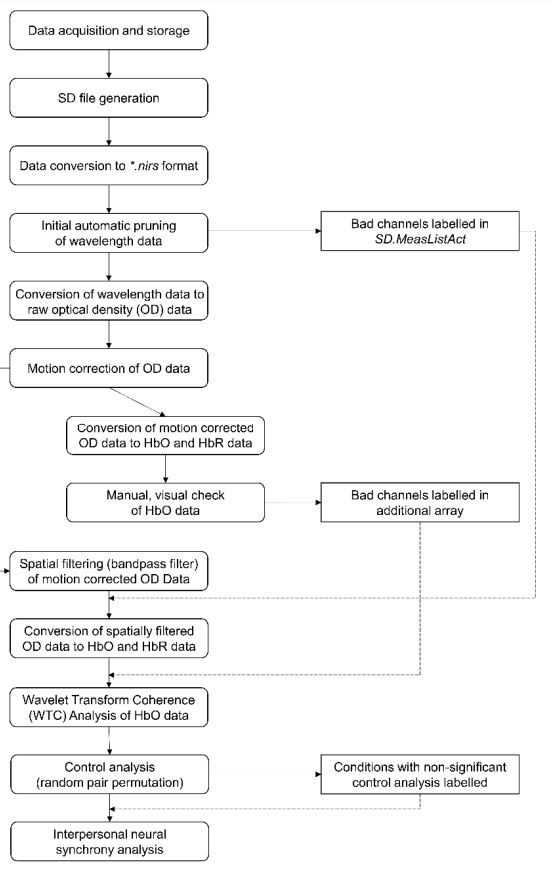
5. Which data analysis steps does fNIRS hyperscanning involve?
There is no unified pipeline for fNIRS hyperscanning data analysis available to date. Data analysis procedures differ as a function of the fNIRS devices themselves as well as previous experience of the labs within which fNIRS hyperscanning is performed. Furthermore, for each individual data analysis step, there are several different feasible options and the order in which they are applied can also vary. Nonetheless, when analysing fNIRS hyperscanning data, the following procedures should be employed at some point or another.
Depending on the fNIRS device manufacturer and data analysis software, the raw wavelength data from the measurement device may need to be converted to a specific data format readable by the used software / toolbox (for example, *.nirs). For subsequent analysis steps, the wavelength data then usually is further converted into raw optical density data(OD).
For automatic bad data exclusion (which is objective and especially useful in case of large data sets), a procedure assessing the expected data range, signal-to-noise ratio and/or the range of interoptode distance can be employed (i.e., enPruneChannels function in HOMER2/3 – for an explanation video, see here).
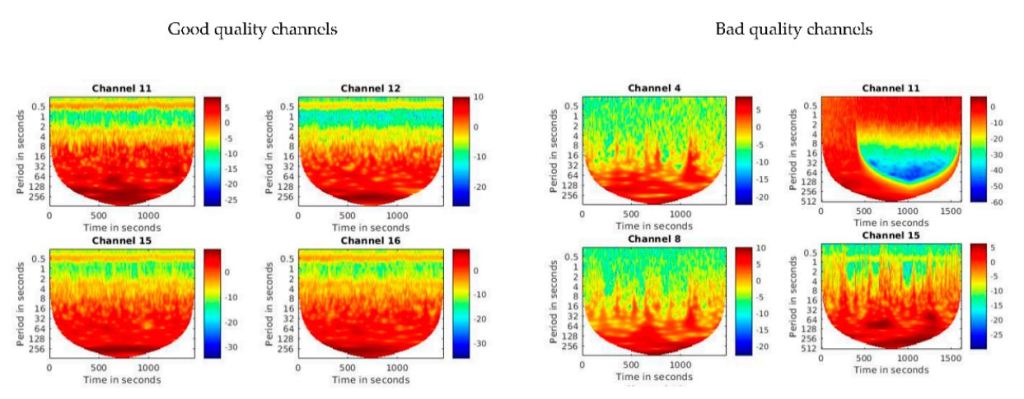
Additionally, we recommend a manual visual data quality check – see Figure “Manual Visual Data Quality Check” below (taken from here). One way to perform such check is to derive WTC plots for each individual measurement channel per participant and to visually inspect the heart band. The heart band should be found at around 0.5 to 1 period seconds in the WTC plot and be consistent and strong. An unclear or absent heart band usually indicates weak signal or complete signal loss at the particular measurement channel. The WTC plot can furthermore inform about important noise in the data or other issues that occurred during data acquisition. See also “6. How is interpersonal neural synchrony derived from fNIRS hyperscanning data?” below to learn more about WTC analysis and plots.
Another step that should be implemented is spatial (bandpass) filtering to exclude any physiological and other kind of noise, particularly at high and low frequencies. Useful additional reading and illustrations can be found here.
What should also be performed is motion correction and/or additional (physiological) noise removal – see Figure “Motion Correction” below (taken from here). Several options are available to do so, including spline interpolation, principal component analysis (PCA), wavelet thresholding or temporal derivative distribution repair (TDDR). For a recent review of these and additional methods, please see here.

Another option that has emerged more recently is the inclusion of short-distance or short-separation channels (SSPs). Usually, for these SSPs, the emitter-detector distance is 10mm – instead of 30mm for regular channels. For more information and additional reading, please see here. Briefly summarised: “A short separation channel measures solely the extracerebral signals, which includes blood pressure waves, Mayer waves, respiration and cardiac cycles. The signal components of the short separation channel can be seen as the “noise” in the signal of the long channel. By removing these components from the long channel, you can minimise this noise.” At the same time, this procedure may not always be trivial because additional steps – besides a simple “removal” or short-separation signal – may be necessary, as explained here.
For subsequent analysis, the processed data then needs to be converted to HbO and HbR values.
The above data analysis steps can either be performed by using the fNIRS device manufacturers’ own software. Alternatively, researchers can employ: HOMER2/3 in MATLAB, SPM for fNIRS in MATLAB, Brain AnalyzIR in MATLAB, Fieldtrip in MATLAB and / or MNE in Python.
We recently suggested one possible pipeline for fNIRS hyperscanning data analysis comprising the above steps in a particular sequence, including freely available sample data from 20 parent-child dyads and links to the required hardware and software (based on MATLAB). The OSF directory can be found here: https://osf.io/wspz4/.

6. How is interpersonal neural synchrony derived from fNIRS hyperscanning data?
One of the most important steps in fNIRS hyperscanning is to calculate INS from the individual brain activation signals. This calculation can be done in many different ways. An excellent review of current methods used in regards to fNIRS more specifically, but also haemodynamic and electrophysiological hyperscanning studies using fMRI, EEG and MEG more generally, can be found here. Below, two commonly used approaches in fNIRS hyperscanning to derive INS, namely intersubject correlation (ISC) and wavelet transform coherence (WTC) analysis, are outlined in more detail.
When using intersubject correlation (ISC) analysis as a measure of INS, a Pearson correlation or robust regression is calculated for specific signal lags and/or frequencies. ISC naturally yields highest positive INS values (i.e., 1) for in-phase signals, lowest negative INS values (i.e., -1) for anti-phase signals and INS values in between for signals more or less shifted in phase. It is also noteworthy that ISC is commonly used to correlate HbO and/or HbR time series themselves and not a derivative of them.
When applying wavelet transform coherence (WTC) analysis, the individual brain activation time series are also correlated using robust regression – like for ISC. However, this only happens after the individual HbO and/or HbR time series were decomposed into different frequency bands and converted to values representing signal strength or power at those individual frequency bands (see Figure “WTC & Phase” below – taken from here). Moreover, WTC analysis yields higher INS values (i.e., closer to 1) regardless of signal lag (i.e., for in-phase signals, signals with phase shifts as well as anti-phase signals), as long as the signals share a consistent temporal contingency. Finally, INS derived from WTC analysis is quite robust to differences in both frequency and amplitude in the source signals.


7. What about control analyses?
When deriving INS by means of ISC or WTC, it is important to keep in mind that there is no implicit INS significance threshold. The obtained INS values from each experiment therefore need to be controlled for spurious correlation. Again, several approaches for such control analyses exist.
A commonly employed approach is to include an active control condition into the experimental design. This enables the comparison of INS between the condition of interest and the control condition – with the hope that INS is significantly higher in the former as compared to the latter. Initially, many fNIRS hyperscanning studies used a passive rest condition as the control condition. While this may work to some degree, there usually is a lot of variance during rest and there may furthermore be some carry-over effects from the preceding active condition. It therefore is better to include another active condition as control condition that is as close to the condition of interest as possible, with some important difference that is thought to be most predictive of INS. For example, an active cooperation condition can be compared to an active individual or competition condition.
While the inclusion of a control condition is helpful, it can still not completely exclude the possibility of spurious correlation. Also, during some experimental paradigms – particularly when examining INS during naturalistic social interaction – there is no adequate active control condition. Therefore, additional and more stringent control analyses have been suggested.
One option is to perform a random pair analysis in which data from a real dyad is compared to a large number of permutations that pair data from one interaction partner with data from various other interaction partners, thereby forming a large number of artificial dyads. The assumption of this permutation analysis is that INS for real dyads should be significantly higher than INS for artificial dyads – which can be statistically tested at various levels (i.e., for each channel, each ROI or as an interaction of channel or ROI with experimental conditions).
Another option is to shuffle and/or scramble the individual data, sometimes even within specific frequency bands. Alternative approaches also propose reversing or shifting the individual data, but these approaches should be employed with caution as INS analysis, especially if derived through WTC, takes into account phase shifts (see “6. How is interpersonal neural synchrony derived from fNIRS hyperscanning data?” above).

8. What else do I need to keep in mind?
When planning an fNIRS hyperscanning study and analysing the data to derive INS, there are several other important issues that need to be considered.
8.1 Task frequency. INS is usually assessed within a limited frequency range deemed most relevant for the experimental task at hand. For some tasks, this can be quite nicely specified – e.g., when there is some sort of external control, such as a given stimulus presentation regularity. For other, and especially more naturalistic tasks this is not feasible, and INS is thus usually derived from a much broader range of frequency bands. The task frequency range with the highest signal power can, of course, be determined from the data post hoc, but this should not be used in a circular manner – i.e., to pre-select and thus cherry-pick the ideal task frequency range for the reported statistical analyses after the data has already been acquired.
8.2 Task duration. INS derived from fNIRS hyperscanning data usually represents an average across the duration of a specific task. Common task durations are in the range of two to five, but can last for up to ten or more minutes. While there is no absolute limit for task duration on the upper end, there is an analysis-imposed limit at the lower end. This comes from the fact that reliable INS estimates require at least two to three complete signal oscillations at the respective signal frequency. For example, if INS is to be assessed for a task (or part of a task) lasting 30 seconds, the upper task frequency limit should be chosen at a signal period of 10 to 15 seconds (0.067 to 0.1 Hz).
8.3 HbO and/or HbR data. There is no consensus to date on which chromophore data should be analysed and reported. However, a recent systematic review identified that most included fNIRS studies analysed and reported either HbO data alone or HbO and HbR data combined, but much fewer included studies analysed and reported HbR data or the HbR-HbO difference data only. The review also summarises the current perception that HbO data appears to be more sensitive to cerebral blood flow (CBF) changes, has better sensitivity to detecting task-evoked changes and is more strongly correlated with the (fMRI) blood oxygenation level dependent (BOLD) response than HbR. Nonetheless, the recommendation is that HbR data should at least also be analysed and reported, with some authors suggesting to additionally check for the presence of a negative correlation between HbO and HbR.
8.4 INS between corresponding single channels / ROIs versus between every possible channel pairing. Many fNIRS hyperscanning studies assess INS between corresponding single channels. For example, for a given number of channels C per participant P in a dyadic setting, INS is examined for C1P1-C1P2, C2P1-C2P2, etc. Similarly, in some cases, certain channels are aggregated to form a region of interest (ROI) and INS is then assessed within corresponding ROIs per participant in a dyadic setting as ROI1P1-ROI1P2, ROI2P1-ROI2P2, etc. Such an approach facilitates the interpretation of observed INS patterns as the same channels or ROIs across the dyad will reflect the same underlying neural computation process linked to their relative anatomical location. It nonetheless remains vital to control for the number of channels / ROIs and thus the number of multiple comparisons.
Alternatively, INS can be assessed for every possible channel / ROI pairing across the dyad, e.g., C1P1-C1P2, C1P1-C2P2, C2P1-C1P2, C2P1-C2P2, etc. While such approach is also feasible, again given the implementation of an appropriate control for multiple comparisons, the resulting INS pattern may be harder to interpret – because increased INS may be observed across different brain areas in the social interaction partners.
8.5 Infant / child versus adult participants. When performing fNIRS hyperscanning in infant / child participants, there are some differences as compared to data acquisition in adult participants. Most fNIRS device manufacturers offer special infant / child optodes that are flat and thus more comfortable to wear. Furthermore, inter-optode distances for infants / children are usually shorter and in the range of 23mm (rather than 30mm). Finally, there will be age-dependent differences in parameters during data pre-processing, for example, a different distal path length factor. For an excellent overview, please check out this webinar by NIRx Medical Technologies about “Infant & Child Imaging with fNIRS” with Dr. Susan Perlman.
8.6 Associations between INS and other measures of BBS. Although INS can be examined by itself, it is advisable to acquire at least one additional measure within the greater realm of BBS – e.g., behaviour, physiology and/or endocrinology – and to test for any associations between INS and BBS. INS may furthermore be combined with self-reports and/or semi-structured interviews.
The link between INS and behaviour is important because several contemporary theories about the functional implication of INS are based on considerations of social interaction behaviour. For example, the mutual prediction account of INS explains synchrony in brain activity by a combination of prediction mechanisms concerning one’s own as well as the interaction partner’s behaviour. Another account of interactional synchrony also highlights temporal regularity in interaction partners’ behavioural expressions as key determinant for the alignment of mental processes, in combination with socio-emotional weighting and thus value attribution. It therefore appears vital to show that during a specific task, observed INS is related to social or interaction behaviour (see also “8.7 Correlation versus Causation” below).
More recent studies are also relating INS with other measures of synchrony within BBS besides behaviour. Although theoretically feasible, a practical difficulty in doing so is related to the sometimes very different time scales of the distinct kinds of synchrony. For example, while INS derived from fNIRS hyperscanning typically measures oscillation patterns with medium frequencies, physiology data (e.g., heart rate) has a much higher frequency, while endocrinology data (e.g., secretion of hormones like oxytocin or cortisol) has a much lower frequency. For a given task, it may thus be difficult to find direct associations between INS and synchrony in physiology and / or endocrinology although these processes may indeed be functionally related (for a recent example, see here).
8.7 Correlation versus Causation. As pointed out in a recent perspective paper, one important question within the field of INS concerns the nature of the relationship between synchrony measured on the neural level and social interaction behaviour. Two perspectives are suggested. On the one hand, INS could be interpreted as a mechanism that causally facilitates social interaction (i.e., mechanistic perspective). On the other hand, INS could be seen as a mere consequence of two (or more) brains processing similar information during a social interaction (i.e., epiphenomenal perspective).
Because of the intrinsic correlational nature of (fNIRS) hyperscanning research, where INS as the dependent variable is correlated with social interaction outcomes as the independent variable, causal effects of INS on social interaction cannot be proved. Several alternative approaches have thus been suggested to prove such causality. One approach involves multibrain stimulation, whereby INS is externally induced and thus becomes the independent variable, and the consequences of externally altered INS on social interaction outcomes as dependent variable are subsequently examined. A few other approaches have also been put forward, including multibrain feedback, the “human dynamic clamp” and mathematical modelling, although these approaches are somewhat controversially discussed.
It should also be mentioned here that both the mechanistic and epiphenomenal perspectives are relevant and valid. The question pertaining to the exact causal relationship between INS and social interaction behaviour should therefore only be assessed as one amongst several others.
8.8 More Synchrony is Always Better. Most of the so far conducted (fNIRS) hyperscanning studies aim to find increased INS in association with a specific social interaction task. In doing so, higher INS is usually interpreted with positive interaction processes and/or outcomes – or, in other words, that more synchrony is always better.
It may, however, also be the case that during certain situations, too much INS may become problematic – especially if it is context-inappropriate and/or conveys the propagation of stress and other negative emotional and physiological states. Accordingly, some researchers have described an “optimal midrange model” of interactive contingency in which both lower and higher poles of contingent coordination – and possibly also INS – may become detrimental for social interaction and development. More research is clearly needed to address this important issue.
In one of our most recent studies now published in Developmental Science, we found increased INS in mother-child dyads with mothers who scored higher on insecure attachment representations (but no such association in behavioural measures). We furthermore observed increased INS in father-child dyads who simultaneously scored lower in behavioural synchrony (compared to mother-child dyads). These data suggest that higher INS may, under certain circumstances, also be related to a neural compensation mechanism to overcome decreased, or reach comparable levels in other interactional synchrony elements (e.g., behaviour).